After Israeli attorney Michal Marks had a brain tumor surgically removed, her right side was paralyzed. Even after additional surgeries and intensive physical therapy, she was still unable to blink her right eye.
“My eye was always open,” she says, causing chronic headaches and cornea damage. “I couldn’t be in light, not outdoors or indoors, without covering my eye,” she added. “I didn’t have a normal life.”
Over the course of four years, one specialist after another told her there was no cure for the problem. Medications such as eye drops didn’t help. Just when she thought the situation was hopeless, her friend Assaf Deutsch, a biomedical engineer with a PhD in biophysics, said something that made her laugh: “Michal, what you need is a pacemaker for blinking.”
He wasn’t joking. After all, he reasoned, heart pacemakers have been around for more than half a century.
Physical electronics engineer Shachar Paz, a friend of both Marks and Deutsch, agreed it was high time for an eyelid pacemaker. They found nothing like this on the market, despite the unmet need. In the United States alone, 200,000 people experience facial paralysis every year, some permanently like Marks and others for a few months as a result of Bell’s palsy.
‘It gave my life back to me’
Deutsch and Paz rigged up a “garage prototype” of an eyelid pacemaker. “Immediately after I put it on, I could blink. All the side effects disappeared,” says Marks. “I had no more pain, no more sensitivity to light. It was truly wondrous.”
She was finally able to resume all her regular activities, including driving and practicing law.
“This product gave my life back to me,” she tells ISRAEL21c.
So she and the inventors gave the product its own life by founding NeuroTrigger in 2018 together with physician Hagit Deutsch as medical director. The advisory board includes Israeli and American doctors Barak Azmon, Anat Loewenstein, Julie Schallhorn and Babak Azizzadeh.
They were later joined by Prof. Eyal Gur, chief of plastic surgery at Tel Aviv Sourasky Medical Center, where the first clinical trial was held. Two years ago, they brought on CEO Nikolai Kunicher, who has an MBA and a PhD in microbiology and formerly led Betalin Therapeutics.
How it works
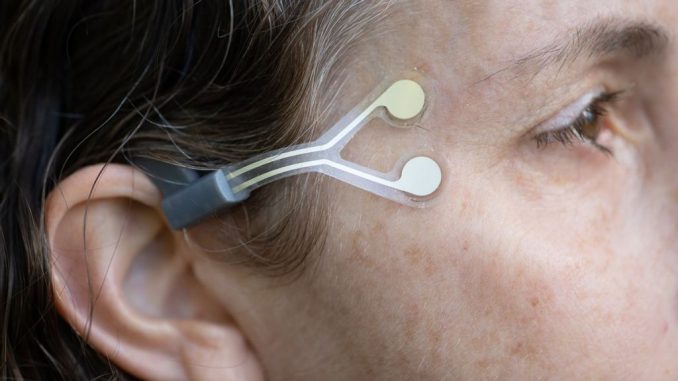
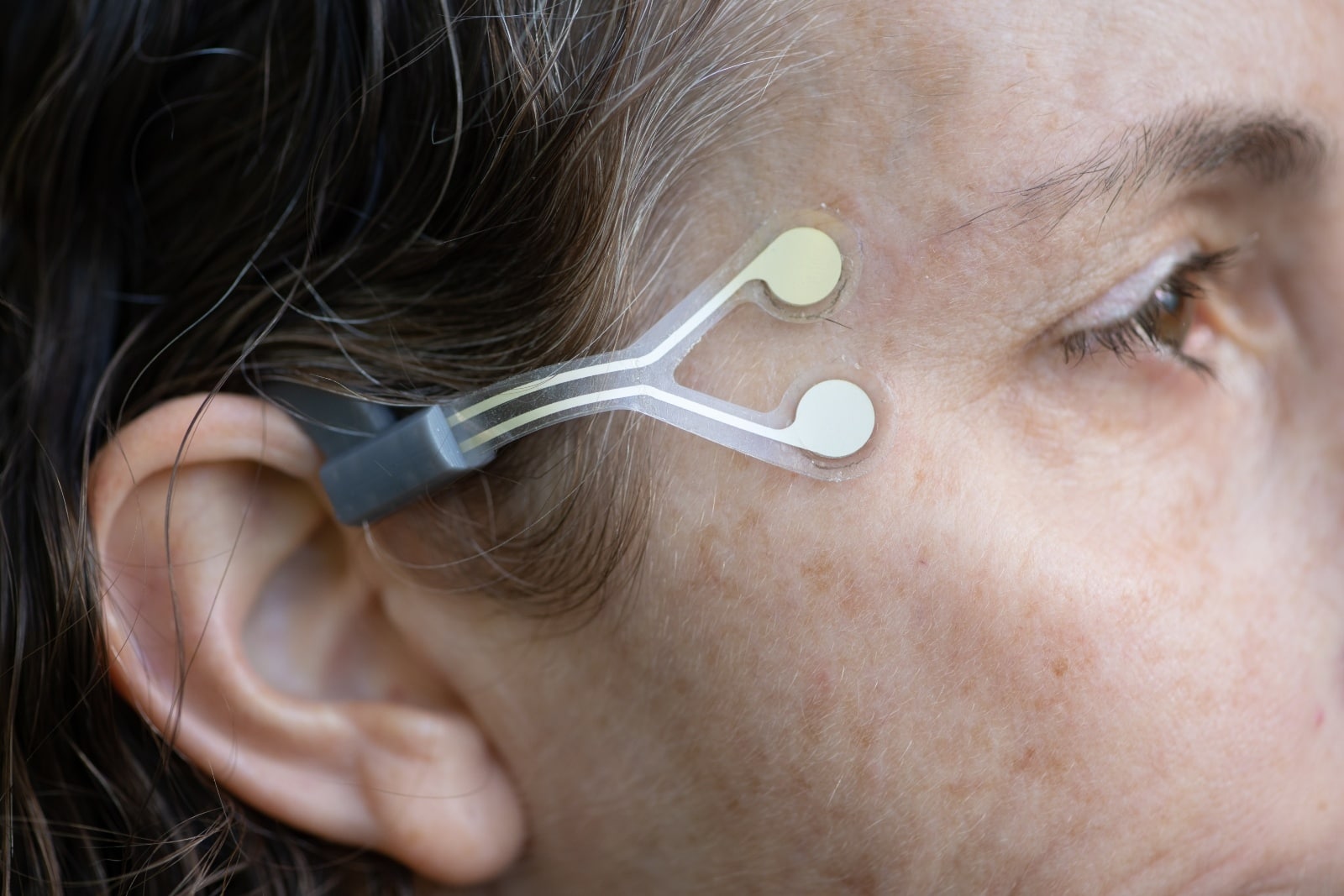
NeuroTrigger’s wearable system is composed of a rechargeable stimulator worn behind the ear, connected by a magnetic flap to a disposable Y-shaped electrode that adheres to the skin next to the eye. Electrical pulses are generated in the stimulator and transmitted through the electrodes, triggering repeated contracting of muscles according to six adjustable individualized parameters set up by a medical professional on a smartphone app.
The stimulator not only “reeducates” the eyelid muscles to blink but also prevents muscle atrophy from disuse, whether the paralysis is permanent or temporary. The stimulator is charged overnight in its own case. Kuchiner explains that when people with paralyzed eyelids go to sleep, they must close the eye with a special tape.
Patented in the US and Europe with more countries coming soon, the technology is “not trying to cure the paralysis but rather to get rid of the side effects,” Kunicher tells ISRAEL21c.
Awards and FDA approval
NeuroTrigger won several grants from the Israeli Innovation Authority and the top prize of $100,000 in the 2019 Merage 45+ Entrepreneurs’ Competition. Last May, Kunicher presented the product at a convention of ophthalmologists in San Diego. “A long line of them were waiting to talk to me,” he says.
In June, the company received an Israel-U.S. Binational Industrial Research and Development (BIRD) Foundation grant to work with Rand Eye Institute in Florida on clinically validating the eyelid pacemaker in the United States. Additional clinical validations will take place in the spring in Israel and the United Kingdom through the support of the UK-IL Pilot program.
In November, NeuroTrigger got 510(k) premarket US Food and Drug Administration clearance of the device, an initial stage in the regulatory approval process. Results of the upcoming validation trials will be used to submit an application for the next stage of FDA approval for specific uses, marketing and insurance reimbursement.
Looking toward CE regulatory approval in Europe, NeuroTrigger recently was accepted into The Medical Forge accelerator in Leipzig. “We are starting meetings with health organizations in Germany to understand the needs of patients and insurers there.”
Based in Ra’anana, the company has five employees and works with a team of subcontractors. In 2023, it closed an investment round from private investors. Another round is planned for early 2024. “It’s been a long and challenging process,” says Marks, “but now we are closer to getting this product to the many people who can benefit from it, who could finally leave the house and live a normal life.”
While the initial market will be people with permanent or temporary facial paralysis, Kunicher and the company’s scientific advisers are already thinking about extending the market to Parkinson’s disease patients and people with dry eye syndrome. The stimulator will be sold by prescription, while the electrodes will be sold off the shelf in drugstore chains or online.
Produced in association with ISRAEL21c
